Electrochemical process
An electrochemical process allows the transformation of a substance in another substance of higher added value. It differs from a chemical process that, instead of chemical reagents, the electric current is used to obtain the desired transformation. The operation is performed in an electrochemical cell, sometimes called reactor, wherein the substrate to be transformed is introduced. This substrate is usually dissolved in a liquid can be flowed through by electric current: the electrolyte. It is in this liquid that ends the electrical circuit allowing the current flow by means of two electrodes. An electrochemical process allows two types of reactions: oxidations which involve removal of the electrons to the substrate and reductions which consist of providing electrons.
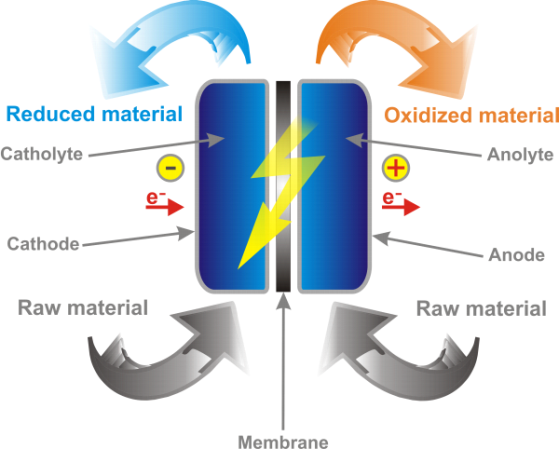
On the industrial side, avoiding the use of chemical reagents is a definite advantage in terms of convenience of operation and safety. Indeed there are no more transportation, storage and handling of chemicals out of the transforming substance and its transformation products. In economic terms, the electron is much more attractive as a redox agent than chemical reagents. In addition, many environmental pressures on the chemical industry are in favor of the implementation of electrochemical processes, since the transformation is done without toxic reactive and with a generally lower level of risk than in most chemical processes. In contrast, processes and technology of electrochemical reactors have greater complexity than chemical reactors, which may result in a higher investment cost. However, the electrochemical reactor technology developed by RedElec SA uses a simple and inexpensive materials configuration. In addition, the characteristics of the material composing the electrode allow precise control of the transformation process and that over a long period of use.
Environmental benefits
To demonstrate the environmental benefits of the electrochemical process, it is necessary to consider the energy efficiency and reductions in greenhouse gas emissions, but also other indicators. To do this, the method of LCA is used. It analyzes the effects exerted by the flow of materials and energy on the environment. The following example is used to evaluate the environmental impact of the electrochemical method proposed by RedElec compared with the traditional chemical process for preparing a dye bath in the denim industry.
The study was based on data provided by ecoinvent, which is the global leader in scientific data for LCA. The characteristics of the products used for the preparation of the dyeing bath were collected by different institutions in accordance with the quality guidelines of ecoinvent. These data allow comparing the electrochemical process and the traditional chemical process using different evaluation methods: the cumulative energy demand, the Eco-Points and the Eco-indicator 99.
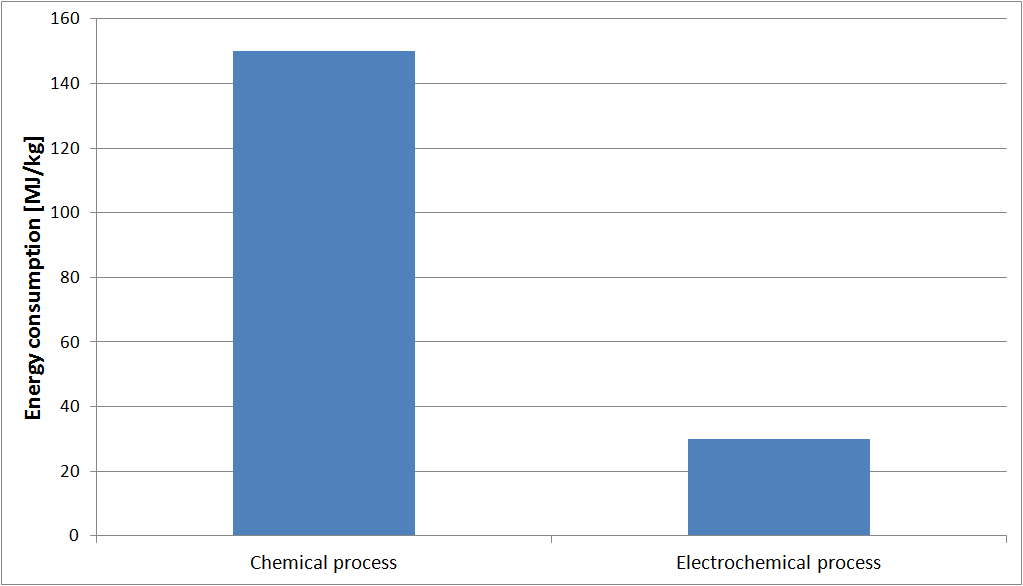
The analysis of the cumulative energy demand is used to calculate the total amount of primary energy used in the manufacture of a product. In this case, the manufactured product is a dyeing bath for the denim industry, prepared according to the electrochemical method of RedElec Technology SA, or using the traditional chemical method.
As shown in the following table, the cumulative energy demand is five times lower with the electrochemical method than with the chemical method.
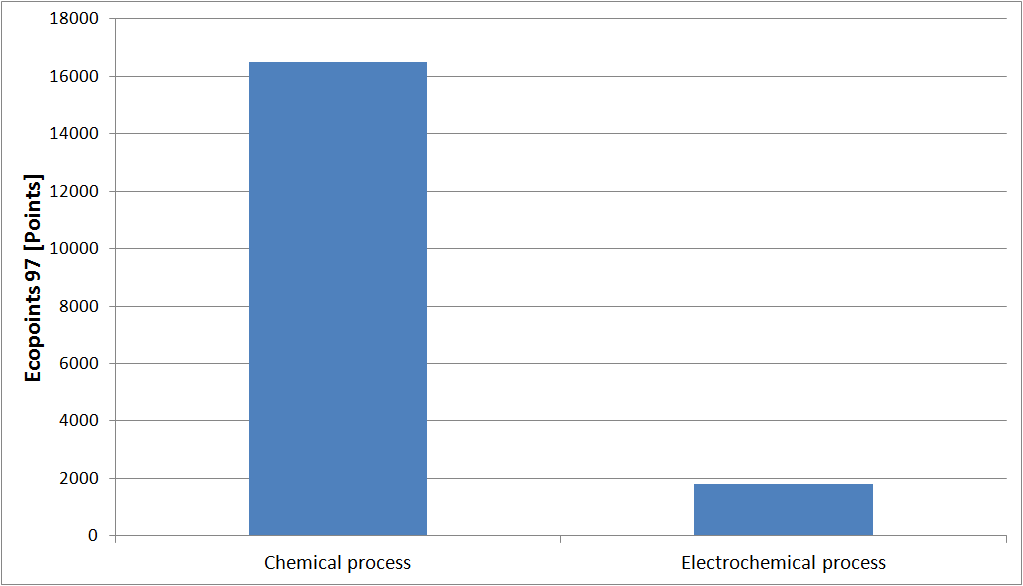
Eco-point is a balancing approach of all impact factors. Points are awarded for each release or consumption, according to the Swiss environmental objectives. The objective is to reach a single score that defines the total impact of a process on the environment. According to this weighting method, the environmental impact of the electrochemical process is ten times lower than the chemical process.
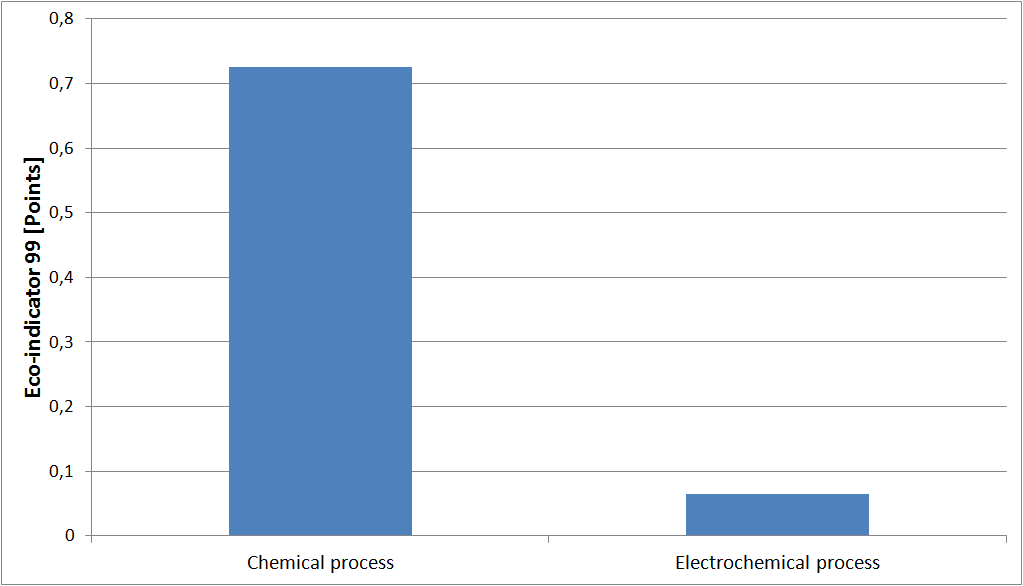
Another weighting method commonly used internationally is that of the Eco-indicator 99. Here, each consumption or rejection is translated using indicators in terms of contribution to an environmental problem. This method, developed at the request of the Ministry of Environment of the Netherlands (VROM) is often used to analyze the life cycle of a product. Unlike the Eco-points method, that evaluates the amount of elementary streams exchanged with the ecosphere, the Eco-indicator 99 method evaluates the effects of a product or a process for each impact category.
In this example, this method shows a decrease of a factor of ten of the overall environmental impact of the electrochemical process compared to conventional chemical process.